Critical Challenges in Manufacturing and Operations
Running a smooth and cost-effective operation is essential for business success. However, many industries face persistent challenges that increase costs, reduce efficiency, and impact overall productivity.
- High Costs and Vendor Lock-in – International vendors often push expensive solutions that require businesses to invest in additional products or services, leading to increased operational costs and reduced flexibility.
- Lack of Real-time Traceability – Poor tracking systems result in delays, miscommunication, and inefficiencies in inventory management, production workflows, and order fulfillment. Without real-time insights, businesses struggle to monitor progress effectively.
- Regulatory Compliance Complexities – Strict industry regulations demand meticulous documentation and adherence, making compliance a resource-intensive and time-consuming process. Failure to comply can result in legal penalties and reputational damage.
- Limited Customer Support – Many solutions offer inadequate customer support, leaving businesses without timely assistance when technical issues or urgent operational challenges arise.
- Manual Processes Hindering Productivity – Dependence on paperwork slows down workflows, increases operational costs, and limits productivity, making it harder for businesses to scale efficiently.
- Human Errors Leading to Financial Losses – Manual data entry and disconnected systems lead to frequent errors, causing miscommunication, compliance failures, and costly rework.
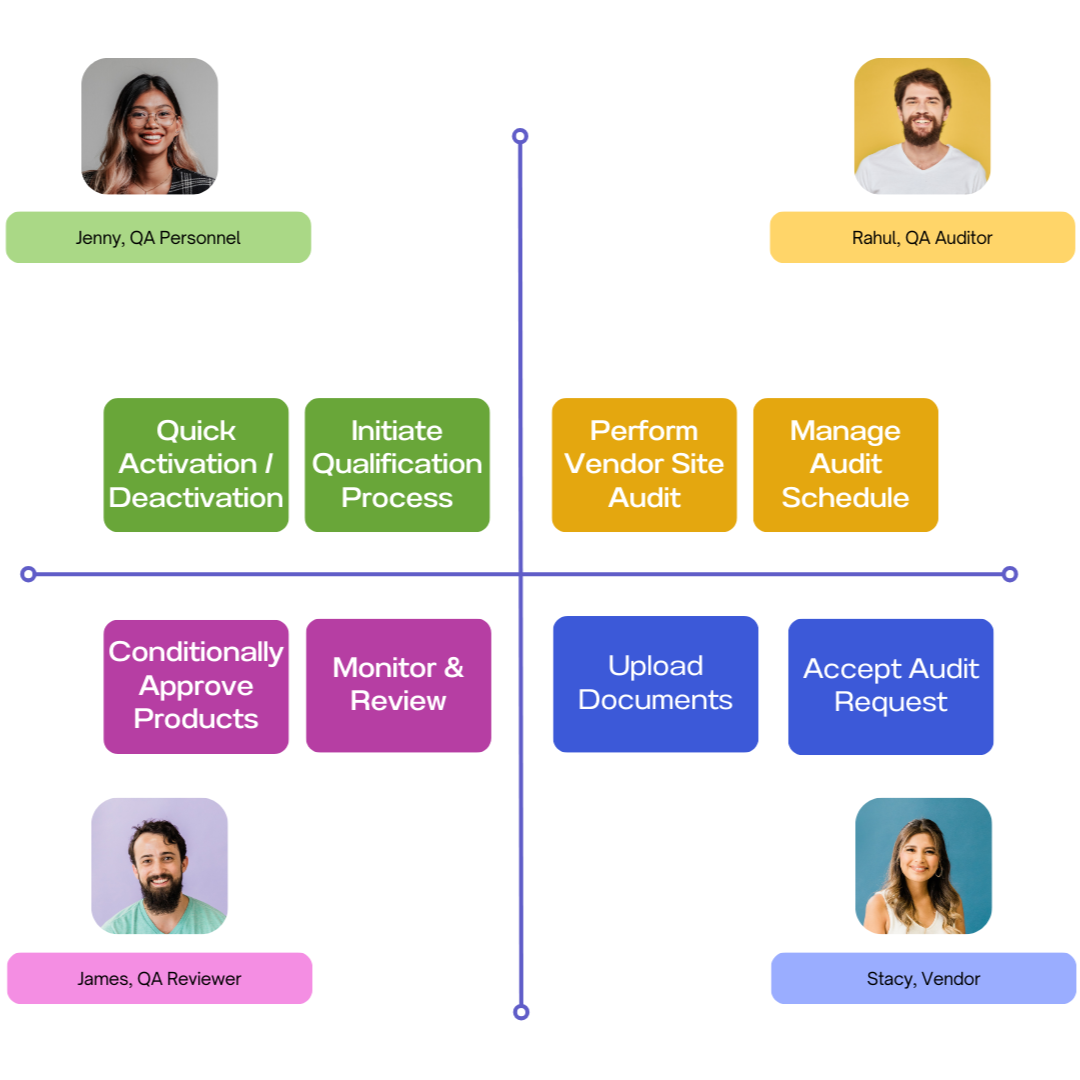
Vendor Qualification
Vendor Qualification ensures a streamlined approval process with customizable workflows, multi-level approvals, audit management, and a Vendor Portal for secure communication and document handling.
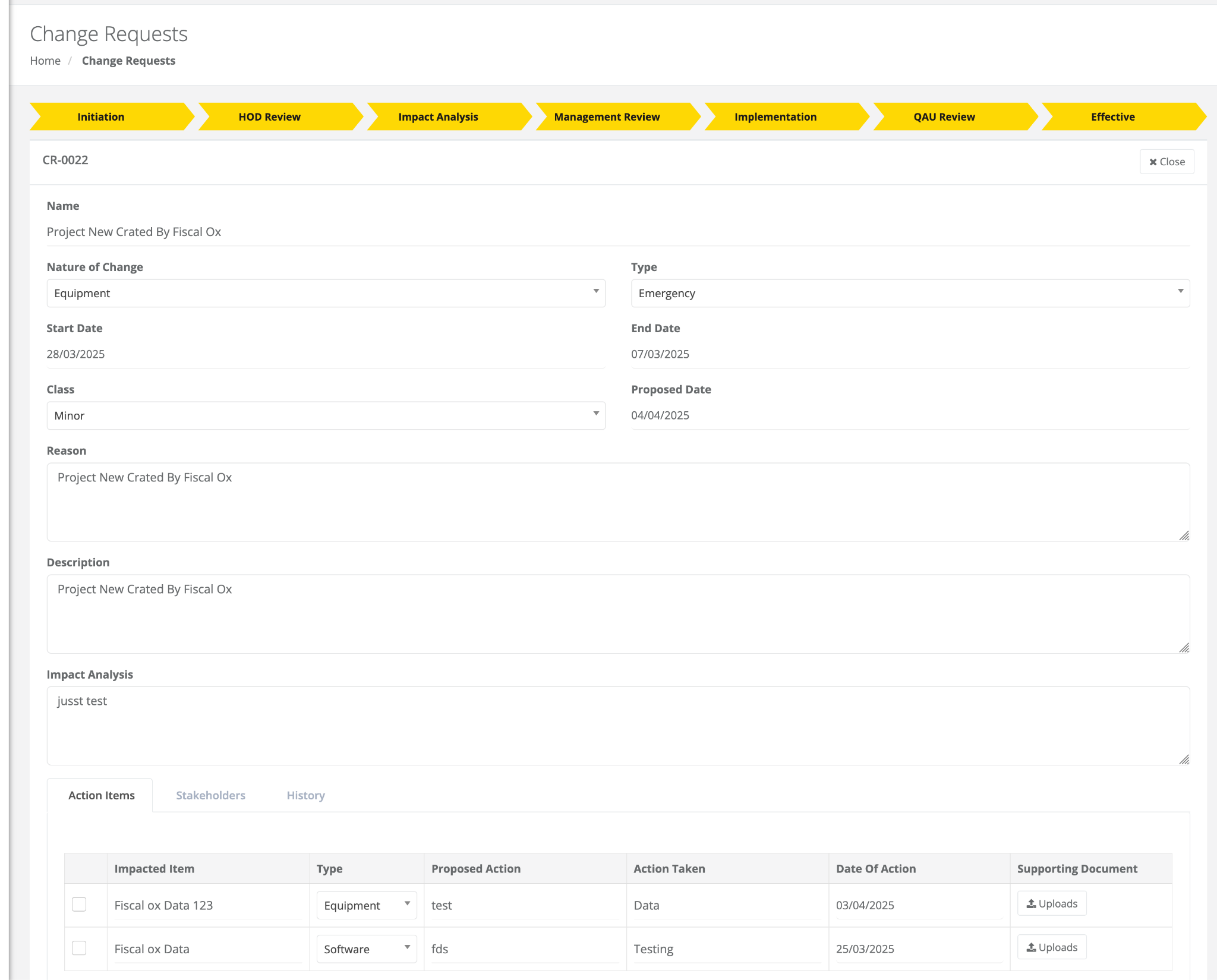
Change Control
Change Control ensures a structured and compliant process for managing changes with customizable workflows, multi-level approvals cross-department collaboration, and a complete audit trail. It enables secure document handling and real-time tracking of action items to meet regulatory standards.
Complaints
The Complaints Management system streamlines issue resolution with customizable workflows, multi-level approvals, and real-time tracking. It enables direct customer acknowledgments, audit management, and vendor collaboration through a secure portal for communication and document handling.

Non-Conformance
Non-Conformance Management ensures effective handling of incidents, deviations, and compliance issues with customizable workflows and multi-level approvals. It facilitates cross-department collaboration, vendor qualification management, audit scheduling, and secure communication through a Vendor Portal.
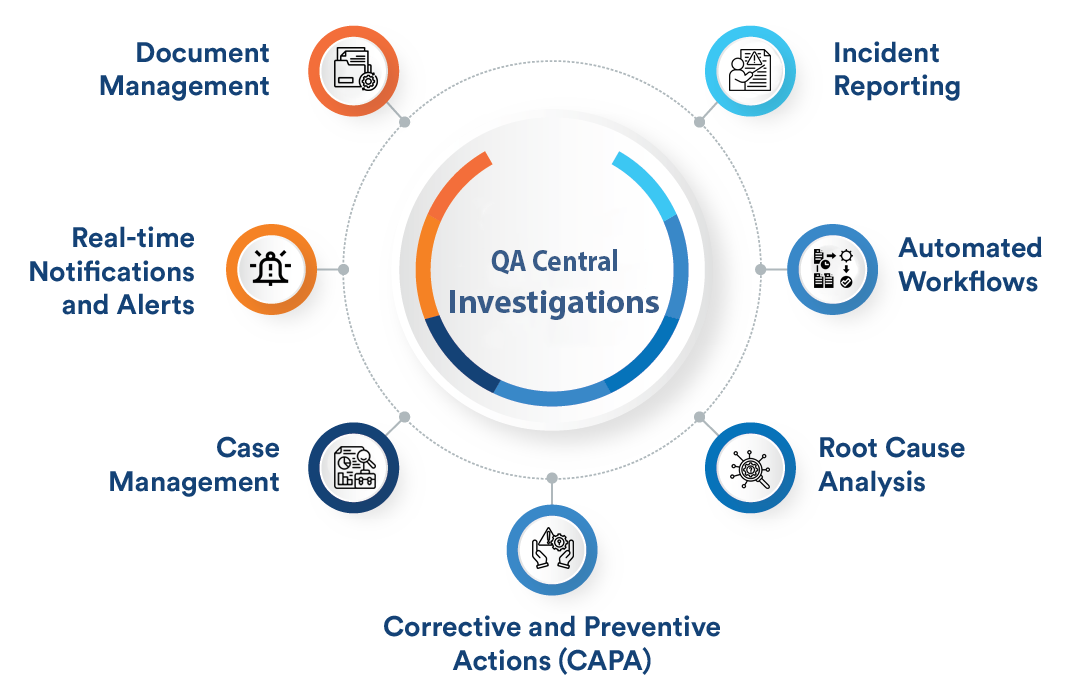
Investigations
Investigations Management streamlines root cause analysis with customizable workflows, multi-level approvals, and real-time collaboration. It includes configurable alerts, supports methodologies like Fishbone and Five Whys, and ensures structured case management for thorough investigations.
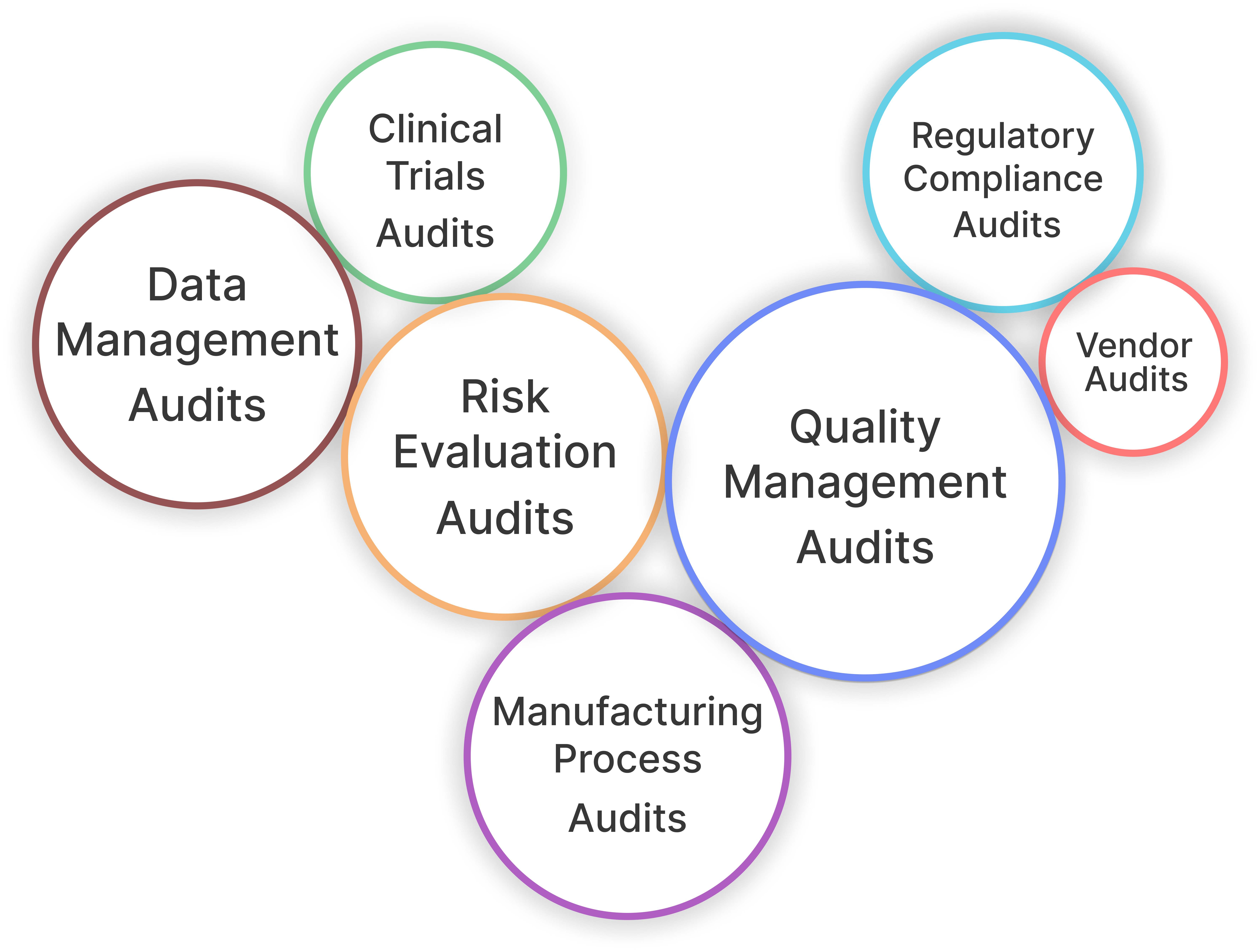
Audits
Our Audit Management System is designed to streamline your compliance processes while enhancing productivity and reducing costs. With Fiscal Ox's Audit Management, you can simplify compliance, reduce manual effort, and ensure your organization meets regulatory standards efficiently.