Pre Clinical Research Modules
We offer smart, streamlined tools to manage toxicology, pharmacokinetics, and safety studies—all before clinical trials begin. Accelerate discovery with organized data, compliance-ready reports, and seamless lab integration.
Study Management
Central hub for designing, planning, and tracking preclinical animal or analytical studies. It manages protocols, study timelines, dosing schedules, and regulatory compliance, ensuring each study aligns with predefined configurations.
Projects & Milestones
Enables oversight of multiple concurrent studies by organizing them into projects with defined milestones. Tracks progress, resource allocation, and timelines to ensure studies meet key deliverables and review checkpoints.
Laboratory & Analysis
Handles sample processing, test execution, and data collection for biological, chemical, or pathological analyses. Integrates with lab instruments and ensures traceability and standardization of data for GLP compliance.
Vivarium Management
Manages animal housing, breeding, health records, cage assignments, and environmental monitoring. Supports IAEC/IACUC compliance and ensures ethical treatment and traceability of all animals used in studies.
Facility Management
Oversees operational infrastructure such as equipment calibration, sanitation schedules, access control, and facility zoning to maintain a compliant and safe research environment.
Reports & Dashboards
Provides real-time insights into study performance, animal welfare metrics, lab results, and operational KPIs through customizable dashboards and regulatory-ready reports.

Efficient Data Storage
Centralized Animal Study Data
All data related to animal studies, such as dosing records, observations, and test results, are stored in a single, centralized system. This eliminates data silos, making it easier for research teams to access and analyze information quickly.
Regulatory Compliance
GLP Standards Adherence
The module is fully compliant with Good Laboratory Practices (GLP), which are essential for pre-clinical studies to ensure the integrity and reliability of data submitted to regulatory agencies like the FDA.

Seamless System Integration
LIMS Integration for Efficiency
The module integrates with existing Laboratory Information Management Systems (LIMS), enabling real-time data sharing between the pre-clinical ERP and laboratory operations for improved workflow efficiency.
Features
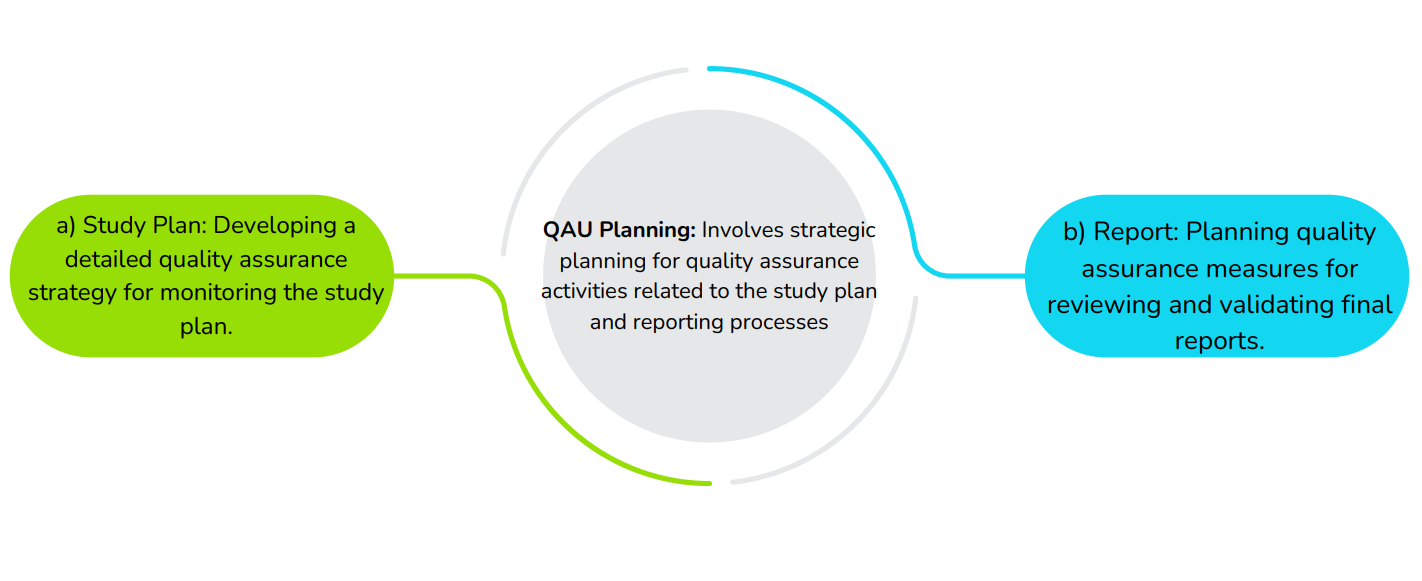
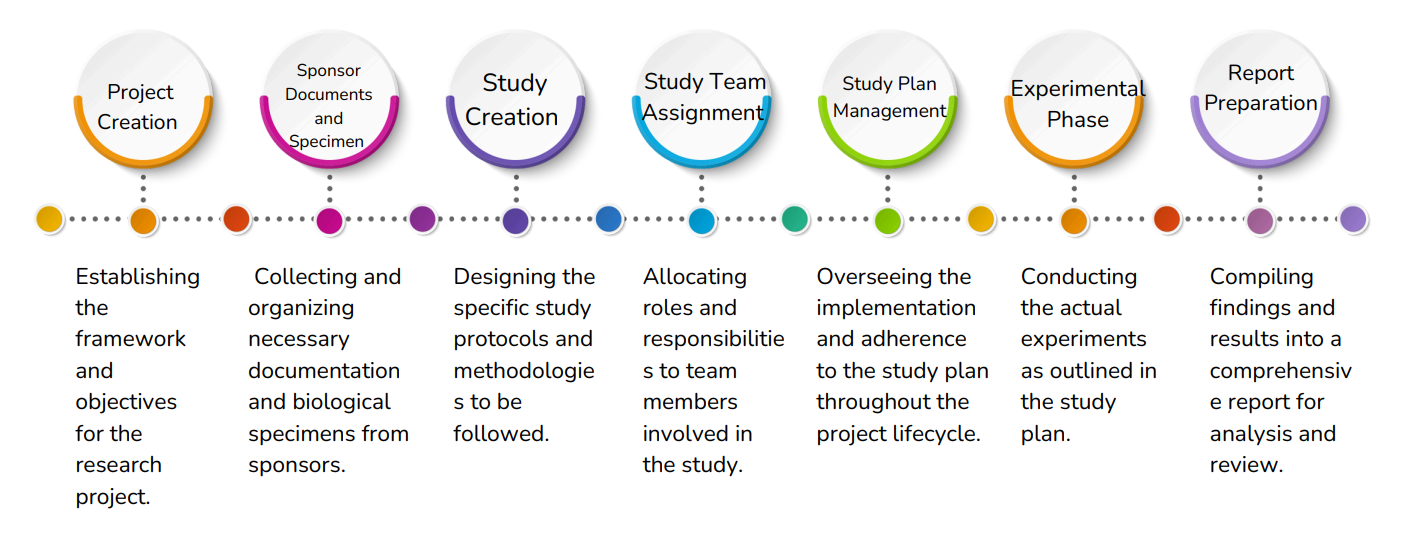
Project Management
Involves organizing and executing research projects, including creating, documenting, assigning teams, managing study plans, executing experiments, and preparing reports.
Contact Us
Send us an email or a WhatsApp message for fastest response.
Contact Info
Phone | Whatsapp: +91 7778091350
Email: info@fiscalox.com
Innovation Center (Head Office): 341 SWC Hub, Vasna-Bhayli Main Road, Bhayli, Vadodara, Gujarat 391412
Regional Office (Goa): TF/11, Third floor, Janaki Sadan 3, Valshi, Mapusa Road, Bicholim, Goa 403504
Regional Office (Hyderabad): T-Hub, Plot No 1/C, Sy No 83/1, Raidurgam, Knowledge City Rd, panmaktha, Hyderabad, Serilingampalle (M), Telangana 500081
Regional Office (Chennai): Coming Soon